By: Jyoti Sharma, Content Marketing Specialist, Catalent Pharma Solutions
The results from a recent drug delivery landscape survey1 confirm the key challenges faced by formulation scientists and R&D managers. Safety, efficacy, bioavailability and solubility issues dominated as the most significant factors in developing drug formulations. As per BCS classification, ~70% of new chemical entities (NCE) are class II or IV, either poorly soluble in water, have low cell permeability or both. It takes tremendous effort and investment for pharmaceutical companies to come up with NCEs which potentially can be commercialized into blockbuster drugs. A drug delivery system that can overcome the solubility and bioavailability challenges can result in increased probability of a commercially successful drug.
 |
| Jyoti Sharma, Content Marketing Specialist, Catalent |
The commercial track record and advancements in formulation technologies have made softgel a method of choice for faster path to market for poorly soluble compounds in oral dosage form. The faster release of APIs from softgels and rapid rate of absorption results in enhanced bioavailability and higher blood levels, which is very advantageous when it comes to poorly absorbed oncology, cardiovascular, analgesic and CNS compounds. Improved absorption can potentially result in decreased inter-patient pharmacokinetic variability. Additionally, softgels can create a solid dosage form for APIs that are in liquids, semisolids and powder forms which cannot be formulated into traditional tablets. Softgels can incorporate a range of fill formulations and the choice can be based on API solubility in the fill. Softgel is an ideal delivery vehicle for low-dose APIs as it can ensure dose uniformity, which is a challenge with traditional tablet manufacturing and powder capsulation processes. Softgels are also better suited for cytotoxic and potent APIs, where safe handling is mandatory, and the hermetically sealed Softgel is a natural fit for light- and oxygen-sensitive compounds.
“The technology to deliver higher API concentrations into smaller capsules can improve patient compliance while delivering full strength efficacy with ~30% smaller size,” comments Ronak Savla, post-doctoral associate at the Rutgers Pharmaceutical Industry Fellowship Program. The novel inline printing technology to feature pictures, brand logos, and product names creates an appealing, recognizable and differentiated drug product.
The current softgel manufacturing process was invented by Robert Pauli Scherer in 1932*. The rotary die Softgel encapsultaion process is a single "form, fill, seal" process. Typically, the outer shell is composed of gelatin, plasticizer and water. Initially, cross linking of gelatin with compounds was a concern, but advance technology has mitigated the risk by modifying formulation strategies such as introducing free radicals and anti-oxidants; selecting the optimal gel formula; control of manufacturing process such as proper machine set-up and limiting exposure to light, oxygen and high temperatures.
The vegetarian alternative of gelatin developed in the last decade has resolved concerns of bovine spongiform encephalopathy (BSE) and dietary and religious restrictions. These alternatives substitute gelatin with carrageenan, a red seaweed extract, and a corn or potato starch derivative. These vegetarian shells have shelf life of more than three years and the shell integrity is maintained throughout. Additionally, they undergo thermal transitions at higher temperatures than traditional gelatin shell systems. This allows encapsulation of lipid fills that are semisolid at room temperature and have high melting points. The combination with semisolid fill formulation can also add to the therapeutic profile by enabling extended and modified release of API’s.
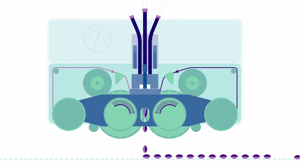 |
Softgel Encapsulation |
Softgels have a track record of commercial success. As a dosage form, they have overcome many limitations to become a versatile and stable oral drug delivery system. Further advancements in softgel technology will solve more formulation challenges and bring better products faster to market. Current and future advancements in softgel technology will enhance the therapeutic profile of challenging compounds, provide optimized path-to-market and add to the bottom line.
 |
| Softgel Capsules |
*Catalent Pharma Solutions is the owner of the RP Scherer softgel technology.
About the Author: Jyoti Sharma is Specialist, Content Marketing at Catalent Pharmaceutical Solutions. She is a scientist turned marketing professional and has a Bachelor’s in Pharmacy. She also holds a Master’s in molecular biology from Worcester Polytechnic and earned her MBA from Penn State University.
References:
- The 3rd annual drug delivery landscape survey was sponsored by Catalent Applied Drug Delivery Institute. For more information, visit www.drugdeliveryinstitute.com
- Soft Capsules, Encyclopedia of Pharmaceutical Technology, Bergstorm, Tindal, Dang; pg 419, Third Edition, October 2006
- Opportunities with Softgel, www.Pharmtech.com, Steven Tindall, August 1, 2014


